shorthand electron configuration|steps to electron configuration shorthand : iloilo This provides the basis for a shorthand notation for electron configurations called the noble gas configuration, which atom consists of the elemental symbol of the . SUPERMEGA an amount equal to the Unauthorized Charges. As a direct and proximate result of the embezzlement by defendants as alleged in paragraph 19, above, SUPERMEGA has been damaged in an amount not less than $60,985.30. SUPERMEGA will seek leave to amend this complaint to state the precise THIRD CAUSE OF ACTION
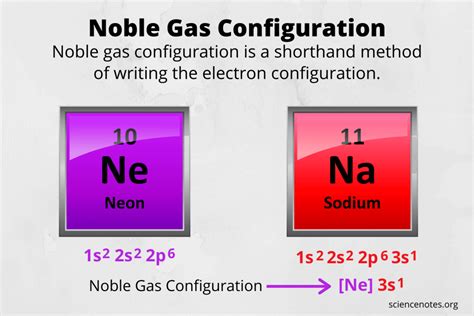
shorthand electron configuration,Learn how to write a noble gas configuration, a shorthand method of writing an atom’s electron configuration. See examples, steps, and a list of noble gas configurations for all 118 elements. Tingnan ang higit paThe noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of the atom’s valence . Tingnan ang higit paFor example, write the noble gas configuration of sodium. 1. The atomic number of sodium is 11, so you know the neutral atom has 11 protons and also 11 electrons. . Tingnan ang higit pa

Mar 23, 2023
This provides the basis for a shorthand notation for electron configurations called the noble gas configuration, which atom consists of the elemental symbol of the . Often times called the noble gas configuration, the shorthand notation will have you writing electron configurations very quickly! In this video, we will cover how to .steps to electron configuration shorthand Find the electron configuration of any element using this tool. Learn the rules, notation, and shorthand method of writing electron configurations. Hun 14, 2015 The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron configurations of the .Learn how to write the noble gas shorthand for any element using the periodic table. See examples, videos, questions and answers about the noble gas configuration and its .Learn how to represent the arrangement of electrons in orbitals of atoms using the periodic table and rules such as Pauli exclusion principle and Hund's rule. See examples of electron configurations for different .
Electron configurations and orbital box diagrams can be written right from the periodic table. The periodic table below, shows the s, p, d, and f-blocks. When reading the periodic table from left to right, one can .
Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which .
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic .shorthand electron configuration Two of the lithium electrons can fit into the 1 s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2 s orbital. Therefore, we write the electron configuration of a lithium atom as 1s22s1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure 2.8.1 2.8. 1 ).
A review of electronic configuration shorthand. View more lessons: http://www.educreations.com/yt/2647375/?ref=ytd Writing electron configurations can be a long and daunting task, until you learn the shortcut! Often times called the noble gas configuration, the shorthand .
shorthand electron configuration steps to electron configuration shorthandThe shorthand electronic configuration is: [Ar] 3d 10 4s 2 4p 1; Even though the 4s is filled first, the full electron configuration is often written in numerical order. So, if there are electrons in the 3d sub-shell, then these will be written before the 4s; Answer 3: A magnesium atom has 12 electrons so its electronic configuration would be The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number . The electron configuration of sodium is 1s22s22p63s1 1 s 2 2 s 2 2 p 6 3 s 1 (Table 2.7.1 2.7. 1 ). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon (Z = 10) ( Z = 10). This provides the basis for a . To write condensed electron configurations (also called abbreviated electron configurations) for elements we first write the full electron configuration for .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .
Electron configurations are shorthand descriptions of the arrangements of electrons in atoms. The electron configuration of a hydrogen atom is spoken out loud as “one-ess-one.” . The electron configuration of Aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Exercise \(\PageIndex{2}\) Using Figure \(\PageIndex{3}\) as your guide, write the .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first .
1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration.

The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. This electron must go into the lowest-energy subshell available, the 3s orbital, giving a 1s 2 2s 2 2p 6 3s 1 configuration.The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\). This provides the basis for a shorthand notation for electron configurations called the noble gas configuration. That is, zirconium is a cation element. Zr – 4e – → Zr 4+. The electron configuration of zirconium ion (Zr 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. The electron configuration shows that the zirconium ion (Zr 4+) has four shells and the last shell has eight electrons.
The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the .
An abbreviated electron configuration uses one of the elements from the last column of the periodic table, which contains what are called the noble gases, to represent the core of electrons up to that element. Then the remaining electrons are listed explicitly. . Electron configurations are a shorthand method of indicating what subshells .
shorthand electron configuration|steps to electron configuration shorthand
PH0 · steps to electron configuration shorthand
PH1 · shorthand electron configuration worksheet
PH2 · shorthand electron configuration for boron
PH3 · shorthand electron configuration calculator
PH4 · how to write shorthand electron configuration
PH5 · how to do shorthand electron configuration
PH6 · electron configuration shortcut
PH7 · abbreviated electron configuration chart
PH8 · Iba pa